These tools development will facilitate isolation of single cells, extraction of their content and their separation first into organelles and then to their components, such as nucleic acids and proteins. Specifically, our group members developed micro-well array devices, which enable live-cell imaging of thousands of primary T cells for extended time periods. We also developed a nanoliter droplet-merging array, constituting a dynamic microfluidic-based system with precise control over droplet composition.
[jcolumns topbordercss=”1px solid #dddddd”] Single Molecule fluorescence and spectroscopy
Drawing of this I-Core’s members experience in this area we will further improve sp-FRET, FLIM and multi-color localization methods. These techniques will be used for in vitro studies of enzyme dynamics, protein-protein complex formation and more. Additionally, we develop an in situ sm-RNA FISH method. By designing libraries of multiple short DNA probes complementary to either introns or exons and coupling these libraries to various fluorophores, bright fluorescent dots will reveal mature mRNA and transcription sites.
[/jcolumns] [jcolumns topbordercss=”1px solid #dddddd”] Live-cell imaging and super resolution methods
Our labs are equipped with state-of-the-art instrumentation for multi-color confocal/TIRF imaging for live cell imaging and STED for super-resolution. These methods will be further developed to support our project. Observation of cellular processes taking place in individual cells in real time will help decipher details of native cellular activity as well as cellular response to external stimuli and cell-cell interactions.
[/jcolumns] [jcolumns topbordercss=”1px solid #dddddd”] Single Molecule force manipulations using optical-tweezers and AFM
We intend to further develop force spectroscopy tools based on high-resolution optical tweezers. These tools will facilitate the study of diverse mechanical processes, such as viral packaging and motor proteins action in solution and in the absence of mechanical constraints. In parallel, we intend to improve our current capacity in force spectroscopy by AFM. The current 1Å spatial resolution will be improved 10-fold and the sampling rate will be increased from 100 Hz to 10KHz. These improvements will stance video-rate 3D imaging of biological processes.
[/jcolumns] [jcolumns bottombordercss=”1px solid #dddddd” topbordercss=”1px solid #dddddd”] Nucleic acids-protein interactions and genome mapping via Nanopores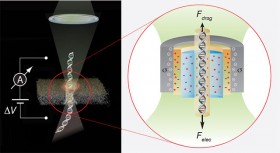
Both protein and solid-state nanopores will be employed to compliment the other techniques for studying genomic DNA methylation, RNA structure, DNA-protein interactions (i.e. mapping of TFs along genomic DNA) and RNA-protein interactions (i.e. the interaction of PABP and eIF complex with mRNA).
[/jcolumns] [jcolumns model=”2,1″] Participating Universities:[jcol/] Funded By:
[/jcolumns][jcolumns model=”2,1″]





[jcol/]


[/jcolumns]